Discovering ACA Pharma
Fully Committed to Innovative Healthcare Solutions
Mastering Our Craft Through Expertise
We are a US-based pharmaceutical wholesaler focused on international exports. Our New York locations hold a wholesale drug license, retail pharmacy license, and DEA license, ensuring compliance with stringent regulatory standards and enabling effective management of pharmaceutical logistics and distribution. Our extensive experience and unique perspective enrich our understanding of the challenges and opportunities in delivering advanced treatments to patients worldwide.
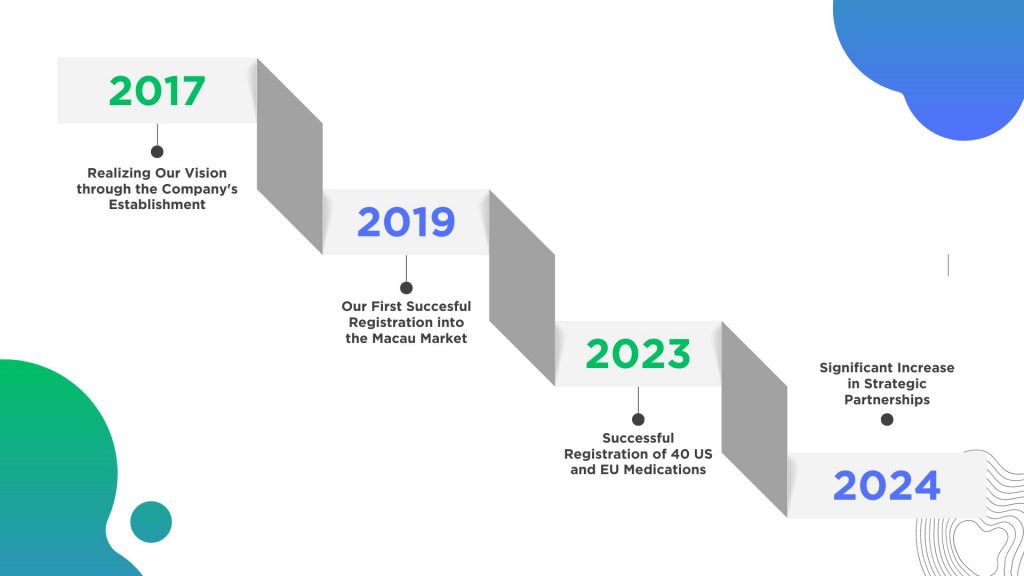
Proven Track Record
Proven Performance of Success and Impact:
- Last year alone, our team successfully registered more than 40 US and European medications, including multiple orphan drugs.
Powered by Partnerships:
- Our office in Macau is also a proud partner and supplier to China Resources Pharmaceutical Group, one of the Fortune Global 500 companies.
- This collaboration significantly enhances our distribution and logistics capabilities across China, ensuring extensive coverage for rare disease treatments nationwide.
Our Distribution Expertise
As a licensed wholesale company based in New York, ACA Pharma specializes as the exclusive distributor for Macau, Hong Kong, Singapore, and China. We streamline market entry by managing direct purchasing arrangements with manufacturers, ensuring swift and efficient distribution.
Our Preferred Relationship
Our preferred relationship involves operating as a Contract Sales Organization (CSO), where we are responsible for the registration, commercialization, distribution, and logistics.
Factory-Authorized Exporter Model
Our business model operates as a Factory-Authorized Exporter. We establish direct accounts with the factory and are authorized to export products to Hong Kong, Singapore, Macau, and China. This ensures efficient and compliant market entry.
IP Protection
ACA Pharma acts as your exclusive overseas wholesaler, market and distribute your product in the approved regions, ensuring your IP remains with you. The manufacturer can continuously benefit from the entire commercial profits permanently without any additional investment.
Immediate Return on Your Investment
After Macau registration, you will immediately receive your first order, and the order volume will continue to increase. All orders are prepaid, so you can immediately begin to see returns on your investment with continuous and perpetual profits.
Fostering Innovative Collaboration
At ACA Pharma, we offer tailored partnership models to fit diverse product and market needs:
- CSO Model: Ideal for mature or orphan drugs, this model allows partners to focus on production while we manage registration, distribution, and market entry, optimizing efficiency and reducing upfront costs.
- Milestone-Based Agreements: Suited for drugs with substantial market potential, these agreements involve flexible funding and shared risks, supported by our investors to align with strategic market goals.
Our adaptable strategies ensure effective market access and risk management tailored to each partner’s needs.
Innovative Process Highlights
We have incorporated a streamlined process integrating meticulous documentation, swift authorization, and direct purchasing, revolutionizing efficiency and transparency in procurement:
- Documentation and Authorization: We secure all necessary regulatory documents and authorizations, including FDA-registered CTDs, product samples, COAs, and CPPs.
- Direct Purchasing: Utilizing our New York office, we place direct orders post-registration, ensuring a consistent supply chain from production to delivery.
Our strategic approach allows manufacturers to focus on their core competencies while we handle the complexities of market access.
Your local partner on the ground
Let us help you expedite your registration in Macau for China access today!

Why work with us?
- Our business model requires no additional investment from the manufacturer.
- Fully licensed operations in Macau and New York, ensuring adherence to rigorous regulatory standards.
- We have a proven performance of success and impact powered by strong and dependable partnerships.
- We specialize in expediting the registration of both new and orphan drugs in Macau, completing this critical process within just 30 to 90 days.
